CSEC Chemistry: Electrolytes
- JH@Quelpr
- May 15, 2020
- 2 min read
Electrochemistry deals with the relationship between electricity and chemical phenomena. So, if you think about it, elctrochemistry is live chemistry (get it?). Anyway, to understand electrochemistry, you must first understand conductors.
Conductors are materials that allow a current to flow through them. This is the exact opposite of insulators (or non-conductors), which do not allow electric currents to pass through them.
Electrical conductors can be divided into two main groups:
metals and graphite
electrolytes
Metals and Graphite
These conductors conduct in both the solid and liquid states, and they experience no chemical changes while conducting electricity.
When these materials conduct electricity, it is due to them having mobile electrons.

Metals have mobile ions because metallic bonding involves metals becoming cations in a sea of mobile, delocalized electrons.
You can probably recall that graphite has one unbonded electron, which is mobile and capable of carrying electrical current.
These moving electrons are what allow for electricity to be conducted, and the metals/graphite remain chemically unaltered.
Electrolytes
Electrolytes (basically ionic compounds which produce a conductive solution when dissolved in a polar solvent) are only able to conduct electricity in their molten (liquid) state or when dissolved in aqueous solutions. They experience decomposition as a result of conducting electricity.
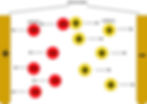
When electrolytes conduct electricity, it is because they have mobile ions, rather than mobile electrons like metals. The positive and negative ions in the compound dissociate (separate), causing the decomposition of the electrolyte.
In the solid state, electrolytes are unable to conduct electricity because the ions are held tightly in a crystal lattice, so they are unable to move. When the electrolyte is melted or dissolved in a polar solvent like water however, the bonds of the crystal lattice are broken, giving the ions the ability to move.
So, we can change our definition of electrolyte to be a molten or aqueous ionic compound which reacts with water to form ions. Electrolytes may also include polar covalent substances which react with water to produce ions.
Electrolytes, much like acids, can be classified as weak or strong based on how completely they ionize.
Strong electrolytes completely ionize in aqueous solution, such as strong acids like hydrochloric acid:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
Weak electrolytes are only partially ionized in aqueous solution, for example weak acids (eg ethanoic acid) and weak alkalis (eq aqueous ammonia)
CH₃COOH(aq) ⇋ CH₃COO⁻(aq) + H⁺(aq)
NH₃(g) + H₂O(l) ⇋ NH₄⁺(aq) + OH⁻(aq)


